Mechanisms of Aging Associated Protein Translation Deficiency Discovered
The teams led by Prof. WANG Tao and Prof. WANG Jie from GIBH collaborated to identify novel mechanisms for regulating aging. The finding was published in Nature Communications with the title "Perturbation of METTL1-mediated tRNA N7-methylguanosine modification induces senescence and aging". The study discovered that METTL1/WDR4-mediated tRNA-m7G methylation was essential in the preservation of proteostasis in healthy cells, which play an important role in the prevention of premature aging. The collapse of proteostasis is one of the most noticeable signs of aging. Various factors contribute to the unfolding and aggregation of nascent proteins during aging.
The occurrence of abnormal protein synthesis, which includes incorrect amino acid integration, protein unfolding, and incomplete polypeptides, increases as individuals age. Simultaneously, the decline in macroautophagy as individuals grow older hampers the degradation of impaired or aggregated proteins. Both factors contribute to changes in the proteome associated with aging. Various interventions, such as mTORC1 inhibitors, caloric restriction, or exercise, have the potential to enhance autophagy and extend lifespan. However, the mechanisms underlying the progression of protein synthesis abnormalities during the aging process remain inadequately understood.
The current study discovered that the METTL1/WDR4 complex is dramatically reduced in three kinds of senescent cells, as well as old mouse liver and kidney tissues. The reduction of METTL1/WDR4 increased protein aggregation and accelerated cell senescence, whereas restoring the complex decreased protein aggregation and delayed senescence. The conditional and ubiquitous deletion of METTL1 notably accelerated aging in mice. However, overexpression of METTL1 significantly promotes the repair of liver and kidney damage caused by cisplatin and doxorubicin.
The results show that hypomethylation of m7G directed some tRNA molecules into rapid tRNA degradation (RTD), resulting in a shortage of tRNA supply, which led to chronic ribosome stalling on specific mRNA codons, inducing the integrated stress response (ISR) and ribotoxic stress response (RSR). The ISR and RSR inhibit global protein synthesis and activate the senescence-associated secretory phenotype (SASP) via phosphorylating eIF2α and p38, respectively. Meanwhile, cell cycle proteins and the Wnt signaling pathway were suppressed at the translational level. The two mechanisms explained how mettl1 deficiency induced cell senescence and aging. The study also confirmed that the transcription factor sp1 controls complex expression. Sp1 may be a regulator of m7G alteration throughout aging.
Previous researches have shown that METTL1/WDR4 was involved in ESC differentiation, neuronal development, and cancer progression. The current study revealed that this complex regulated senescence and the aging process. Furthermore, the investigation also elucidated the upstream signaling pathway responsible for regulating proteostasis during aging, suggesting possible approaches to enhance healthy aging.
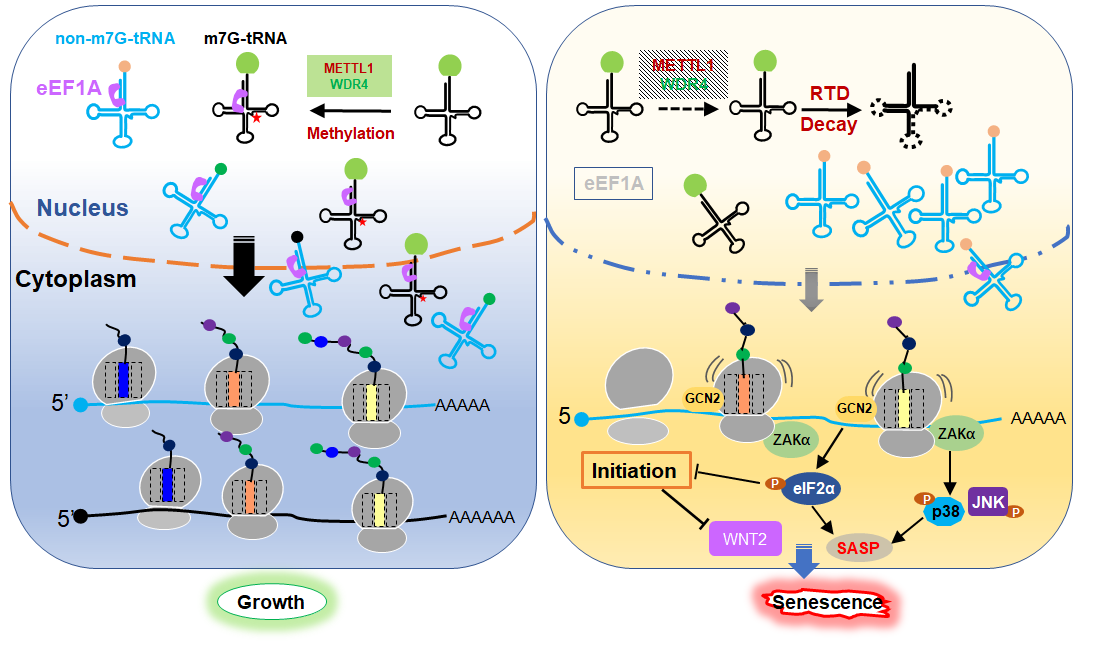
SchematicDiagram of Senescence Mediated by METTL1 Deficiency (Image byGIBH)
Contacts:
TaoWang, Ph.D., Principal Investigator
GuangzhouInstitutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou,China, 510530