Researchers Uncover Novel Mechanism Regulating GPCR-G Protein Signaling from Endosomes
G Protein-Coupled Receptors (GPCRs) were traditionally believed to be activated solely on the plasma membrane, becoming desensitized post-internalization. However, increasing evidence suggestes that some internalized GPCRs retain their activity on endosomes, and their signaling from these compartments has been implicated in various physiological and pathophysiological processes. Regulator of G protein signaling (RGS) proteins were well-characterized as negative regulator of G protein signaling from plasma membrane by promoting the inactivation of Gα subunits. Yet, the mechanism for terminating G protein signaling from endosomes remains largely unknown.
Recently, researchers from Guangzhou Institutes of Biomedicine and Health (GIBH) of Chinese Academy of Sciences published a paper entitled"Redox-Modulated SNX25 as a Novel Regulator of GPCR-G Protein Signaling from Endosomes" in Redox Biology. This study revealed that SNX25 regulates GPCR-G protein signaling from endosomes by forming a complex with canonical RGS proteins in a redox-modulated manner. This is the first time a mechanism for terminating G protein signaling from endosomes has been uncovered.
The researchers firstly discovered that SNX25 could bind to several canonical RGS proteins, including RGS2, RGS4, RGS8 and RGS17. Through structural analysis and cell-based assays, they found that these interactions heavily depend on the disulfide bond between a cysteine (C566) in mSNX25 and a cysteine in the N-terminal domain of RGS proteins, and may be regulated by a redox process. Further experiments showed that the SNX25 targets to endosomes, via its PXA or PXC domain. By recruiting canonical RGS proteins to endosomes, SNX25 promotes the inactivation of Gαi/q on endosomes, ultimately inhibiting the signal transduction of GPCR-Gi/q from endosomes. In addition, researches further identified that SNX25/RGS complex negatively regulates Gαi/q signaling from the plasma membrane by recruiting GDP-bound Gαi/q to endosome, preventing its activation on plasma membrane.
In summary, the discovery of redox-modulated SNX25 inhibiting Gαi/q signaling unveils a mechanism for terminating Gαi/q signaling from endosomes, expanding the paradigm in the regulation of GPCR-Gαi/q signaling.
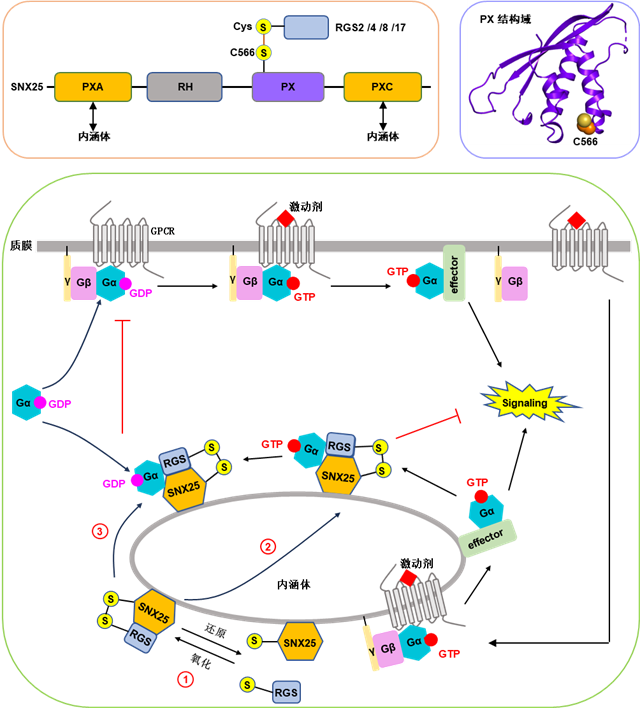
Mechanism on SNX25 regulating GPCR-G protein signaling(Image by GIBH)
Contacts:
Jinxin Xu, Ph.D., Principle Investigator;
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, China, 510530.
Email:xu_jinxin@gibh.ac.cn