Researchers reveal a novel mechanism by which Autophagy regulates the expansion of human myeloid progenitors
In a study published in Stem Cell Reports, the team led by Prof. PAN Guangjin from Guangzhou Institutes of Biomedicine and Health of the Chinese Academy of Science (GIBH, CAS), revealed a novel mechanism by which ATG7-dependent autophagy regulates the expansion of human myeloid progenitors (MPs) during emergency myeloid/granulopoiesis (EM).
Granulocytes are important immune cells in human which play essential roles in immune response, inflammation regulation and tumor development. During systemic infection, the body activates EM to defense against pathogens for rapid replenishing of mature myeloid/granulocytes. During EM process, a rapid cell cycle switching from the quiescent hematopoietic stem cells (HSCs) to highly proliferative MPs is critical. How the rapid proliferation of MPs during EM is regulated remains poorly understood. Previous studies have shown that autophagy plays a pivotal role in mouse granulopoiesis. Due to ethical and sampling limitations, whether autophagy regulates human granulopoiesis remains less known. Therefore, the research team established an EM model based on a previously developed in vitro hematopoietic differentiation system derived from human pluripotent stem cell (hPSCs) to conduct further investigations.
The team firstly showed that peripheral blood mobilized hematopoietic stem/progenitor cells (HSPCs) with ATG7 knock-down or HSPCs derived from ATG7-/- hPSCs exhibit severe defect in proliferation during EM process. And in contrast to wide type autophagy competent ATG7, the mutant ATG7 with autophagy function defect failed to rescue the proliferation defect in ATG7 deficient MPs. These results indicate that ATG7 maintains the expansion capacity of MPs during EM process through its autophagy-dependent function.
Furthermore, the team found that a large amount of p53 protein accumulated in autophagy-deficient MPs, while the mRNA level of p53 did not significantly increased. Which suggested that autophagy may play a crucial role in regulating the stability of p53 protein in MPs. Through co-immunoprecipitation (Co-IP) assay, the team subsequently showed that p53 protein was indeed associated with LC3B, an important adaptor interacting with cargoes in autophagosomes. And p53 protein was also co-localized with LAMP2, the lysosome membrane protein and the co-localization was substantially reduced in ATG7 deficient MPs. These data suggests that autophagy defects lead to a decrease in p53 protein transported to lysosomes for degradation, further validating the mechanism by which p53 protein is degraded through the autophagy-lysosome pathway. Furthermore, knocking down p53 in autophagy-deficient MPs significantly restored their expansion capacity during EM process.
In summary, this study demonstrates that ATG7-dependent autophagy liberates the proliferative capacity of MPs by degrading p53 protein, thereby ensuring MPs expansion during EM process. The study provides new insights into the regulation of human granulopoiesis and offers potential targets for the clinical development of treatments for acute myeloid leukemia.
This research was supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China, and other projects.
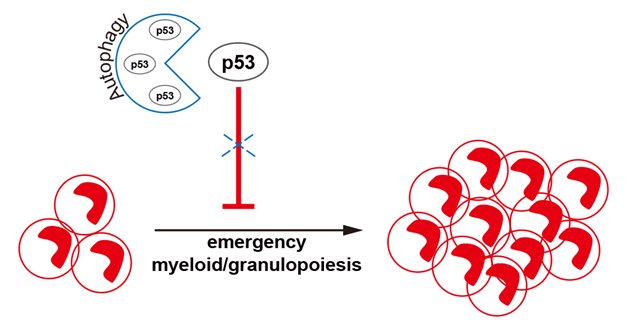
Figure 1. Model for autophagy dependent p53 degradation in emergency myeloid/granulopoiesis.
Contacts:
PAN Guangjin,Ph.D, Principal Investigator;
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou, China, 510530
Email: pan_guangjin@gibh.ac.cn