GIBH Reveals How MARCH7 Regulates Autophagy via Ubiquitinating ATG14
Recently, the team led by Dr. ZHANG Xiaofei from the Guangzhou Institutes of Biomedicine and Health of the Chinese Academy of Sciences, published a research paper titled"MARCH7-Mediated Ubiquitination Decreases the Solubility of ATG14 to Inhibit Autophagy"in the journal Cell Reports. The study revealed the mechanism by which the E3 ubiquitin ligase, MARCH7, inhibits autophagy by ubiquitinating and modifying ATG14, leading to the disruption of protein aggregates' degradation through the autophagy pathway.
Autophagy is a conserved and important degradation mechanism in eukaryotic cells, closely related to disorders such as cancer, neurodegenerative diseases, and aging. In recent years, with the advancement of research on autophagy, there has been growing evidence of the significant involvement of ubiquitination, a commonly observed post-translational modification, in this process. However, the complex and precise regulatory network of autophagy remains incompletely understood. Deciphering the autophagy code is still an indispensable part of fundamental research for the coming decades.
In this study, MARCH7, a key protein in the core mechanism of autophagy, was identified through techniques such as proteomics. The study further determined the ubiquitination modification sites and types on ATG14 by MARCH7.
This research provides a basis for the study of ubiquitin chain function and offers new evidence for refining the regulation mechanism of autophagy. It also establishes new directions and research targets for the treatment of autophagy-related diseases.
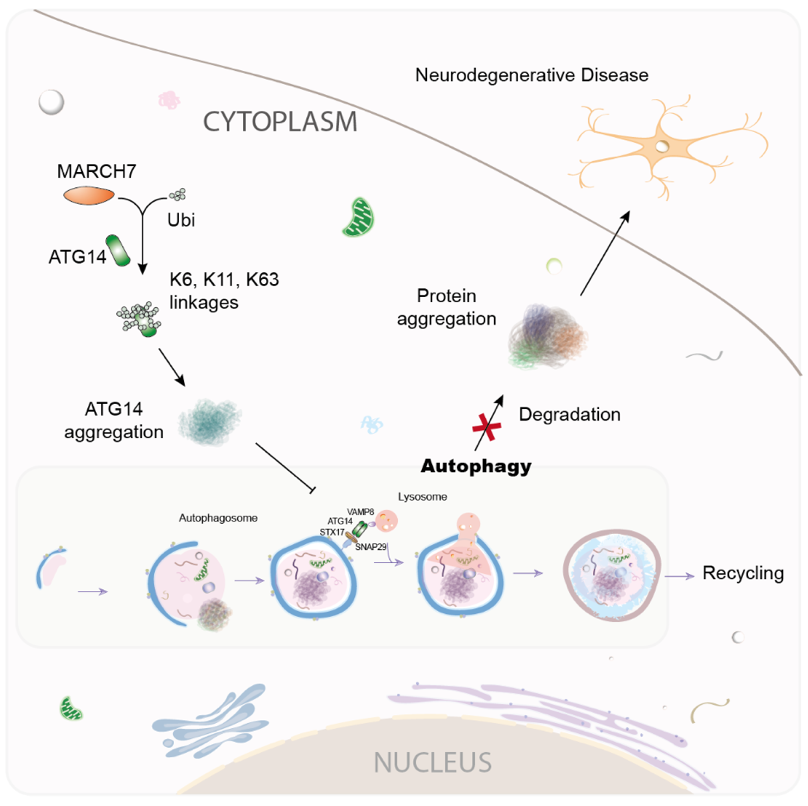
Fig.1 MARCH7 Regulates Autophagy via Ubiquitinating ATG14 (Image by GIBH)