Research Progress
Scientists Reveal an epigenome-metabolome-epigenome signalling cascade mediated by Glis1 facilitating pluripotency induction
Date:Aug 25, 2020
On August 24, Nature Metabolism journal published a latest research titled “Glis1 facilitates induction of pluripotency via an epigenome–metabolome–epigenome signalling cascade” from Xingguo Liu’s group in Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences. The work found that transcription factor Glis1 as a powerful reprogramming factor enables senescent cell reprogramming, and protects the genome stability. The work revealed an epigenome-metabolome-epigenome signalling cascade mediated by Glis1 that involves the glycolysis-driven coordination of histone acetylation and lactylation in the context of cell fate determination.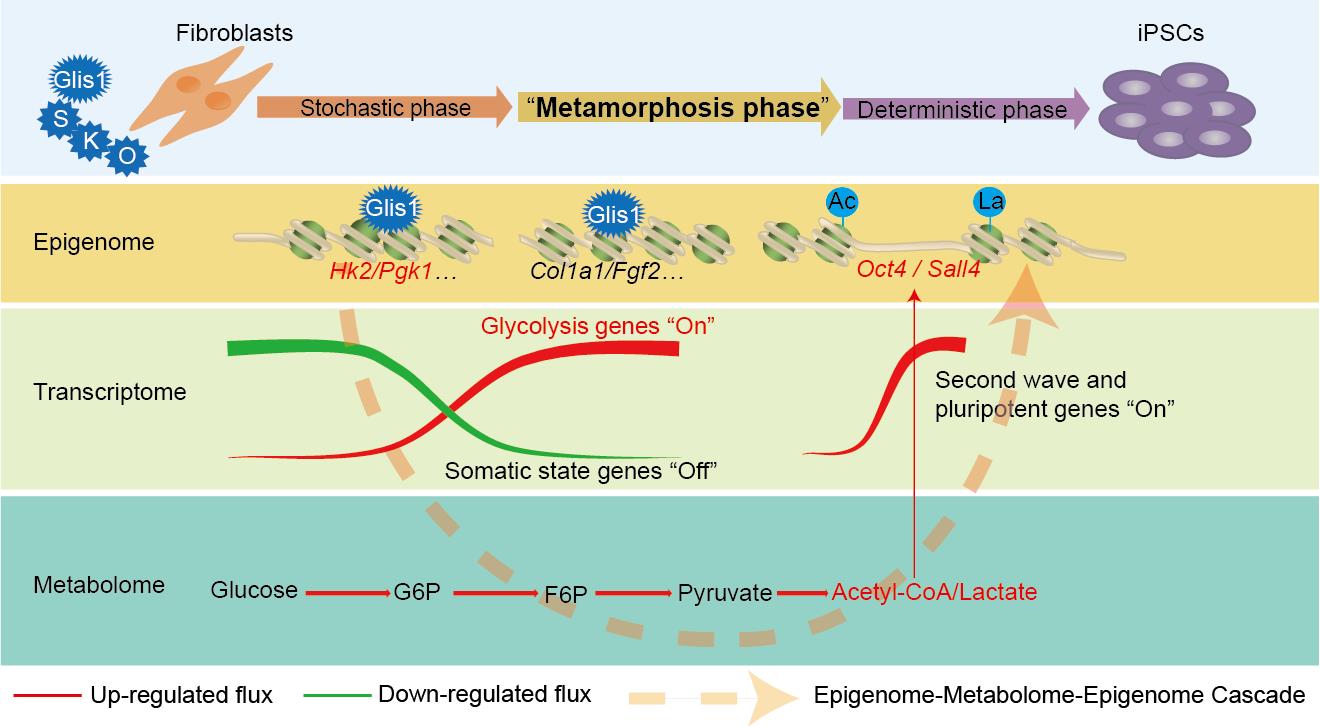
iPSCs hold great promise in regenerative medicine therapy. Furthermore, iPSCs technology offers a unique experimental system to investigate the principles of cell fate determination. Though iPSCs process have been extensively studied, but multi-level routes during reprogramming remain unclear.
Known as the “Fifth Yamanaka Reprogramming Factor”, maternal transcription factor 1 (Glis1) is high in unfertilized eggs and one-cell embryos. Xingguo Liu’s group showed that Glis1 enhances senescent cell reprogramming and maintains the genome stability of iPSCs, suggesting that Glis1 is a powerful reprogramming factor with great promise for future regenerative medicine therapies of older people.
Further, Xingguo Liu’s group revealed an unique three phase (epigenome-metabolome-epigenome cascade) triggered by Glis1 facilitates induction of pluripotency by multi-omics joint analysis. In phase 1, Stochastic phase, this cascade is initiated by the binding of Glis1 to promoters of somatic genes and glycolysis genes at the early stage of reprogramming. Glis1’s binding shuts off somatic gene expression while turning on glycolysis gene expression. Then, in phase 2, Metamorphosis phase, this glycolysis genes expression facilitates the metabolic remodeling from mitochondrial OXPHOS to glycolysis. The upregulated glycolysis produces more lactate and acetyl-CoA, key metabolites linking metabolism and epigenetics. Finally, in phase 3, Deterministic phase, the enhanced lactate and acetyl-CoA level promotes histone lactylation/acetylation and expression of “second wave” and pluripotency genes. Thus, phase 2 acts as a “Metamorphosis phase”, bridging phases1 and 3 by amplifying epigenomic signals through a unique metabolic remodeling consisting of activation of glycolysis without affecting mitochondrial OXPHOS.
Glis1 is not only high in unfertilized eggs and one-cell embryos, but also highly expressed in various cancers under hypoxia conditions. Cancer cells also reply on glycolysis to supply energy. “Our findings regarding the epigenome-metabolome-epigenome cascade mediated by Glis1 might provide hints to the function of Glis1 in these processes as well” according to Dr Liu
It is worth mentioning that this study found that a new type of histone lactate modification produced by lactate regulates pluripotency. This is the first time that histone lactylation modification was discovered in pluripotency acquisition. Lactate, in the opinion article of Nature Metabolism on July 20, is considered that the ugly duckling in this metabolic field is becoming a white swan of metabolic remodeling. The discovery of Dr. Xingguo Liu's team laid the foundation for this emerging direction.
"Butterfly effect": A butterfly in the tropical rainforest of the Amazon River Basin in South America, occasionally flapping its wings, may cause a tornado in Texas, the United States two weeks later," is a chaotic phenomenon of cascading reactions. Transcription The factor Glis1 is like the breeze of the butterfly love flower, triggering a tornado with pluripotency at the whole genome level. This butterfly effect cannot be achieved at the genome level alone. It needs "stones from other mountains"-metabolic levels to connect across borders to form "A trilogy of cascading reactions of the “epigenome-metabolome-epigenome”. “Somatic cells need to rejuvenate and return to the pluripotent state of sperm and eggs, which requires metabolism, a cross-border foreshadowing,” said Dr Liu.
This research was completed in cooperation with Fudan University, and received funding support from the National Key Research and Development Program of China, National Natural Science Foundation of China, Chinese Academy of Sciences, Guangdong Province, Guangzhou City, and Post-doctoral start-up funding.

Contact:
Prof. LIU Xingguo, Ph.D Principal Investigator
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences (http://www.gibh.cas.cn/)
Guangzhou, Guangdong 510530, China
Tel: 020-32015225
E-mail: liu_xingguo@gibh.ac.cn