Chinese Scientists Reveal Novel Membrane Binding and Cargo Protein Recognition Mechanism For Sorting Nexins
Research groups led by Dr. LIU Jinsong and Dr. SHU Xiaodong from the Guangzhou Institutes of Biomedicine and Health (GIBH) revealed that SNX16 regulates E-cadherin recycling trafficking through a unique mechanism of coordinated membrane and cargo binding. The work was published in Cell Press journal Structure on July 13th.
Endocytosis and subsequent protein sorting through endosome network are critical for nutrition absorption, cell-cell adhesion and signaling. Sorting nexins (SNXs), a family of phosphatidylinositol-binding proteins defined by a common PX domain, are involved in endocytosis and endosomal sorting processes. SNX16 is a unique SNX family protein that contains a coiled-coil (CC) domain downstream of the PX domain.
The researchers found that SNX16 regulates the recycling trafficking of E-cadherin. To uncover the mechanism for SNX16 regulating E-cadherin recycling trafficking, they determined the crystal structure of PX-CC unit of SNX16. The structure reveals a unique shear shaped homodimer and a novel PI3P binding pocket in SNX16 that consists of both the PX and the CC domain. They further showed that the PPII/α2 loop, which is generally regarded as a membrane insertion loop in PX family proteins, is involved in SNX16 binding with E-cadherin. Taken together, the researchers postulate a new mechanism for coordinated membrane binding and cargo binding for SNX family proteins in general and provide novel insights into the recycling trafficking of E-cadherin.
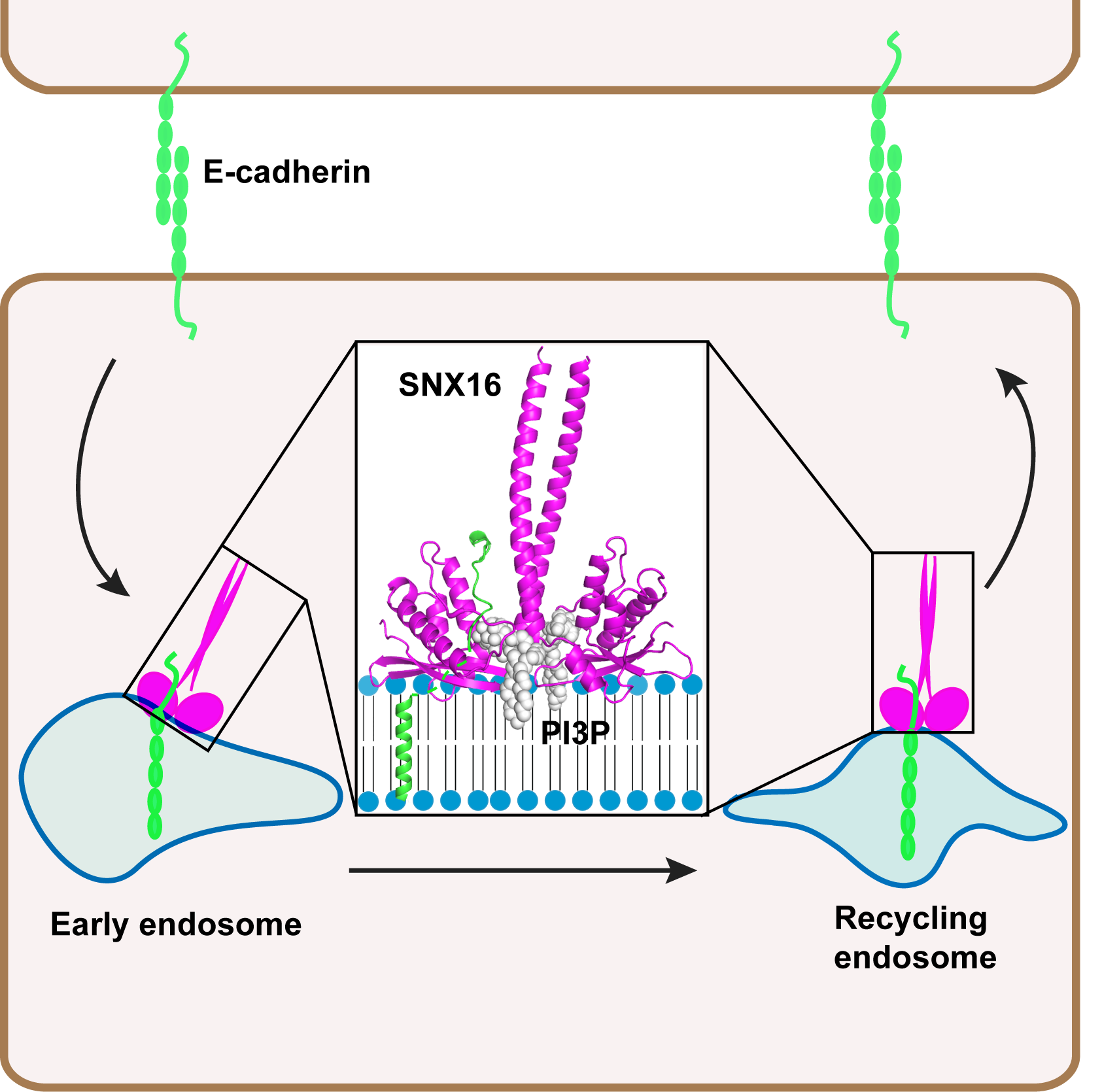
Model for SNX16 regulating E-cadherin recycling trafficking (Image by GIBH)
contact:
XU Jinxin
Guangzhou Institute of Biomedicine and Health (GIBH)
Chinese Academy of Sciences 190 Kaiyuan Avenue, Guangzhou Science Park Guangzhou ( Canton ) 510530, China
E-mail: xu_jinxin@gibh.ac.cn