Scientists at GIBH discovered that Neurod1 can modulate opioid antinociceptive tolerance via two distinct mechanisms
Recently, GIBH scientific group led by Prof Zheng Hui has discovered that Neurod1 regulates the developments of opioid tolerance via a time-dependent pathway through contextual learning and a short-response pathway through antinociception. This new finding has been published on line by Biological Psychiatry (impact factor: 9.773).
Neurogenic differentiation 1 (Neurod1), a basic helix-loop-helix transcriptional factor, was first identified to convert ectoderm into neurons in Xenopus embryos and plays critical roles in the development of the pancreas, cerebellum, and hippocampus. NeuroD1 also functions in maintaining the dendritic morphology of granule neurons. Reducing the activity of NeuroD1 by microRNA-190 (miR-190) or by inhibiting calcium-calmodulin kinase II α reduces dendritic spine stability in primary hippocampal neurons. NeuroD1 has also been identified to regulate adult neurogenesis in the subgranular zone of hippocampal dentate gyrus (DG). Reducing NeuroD1 activity in DG with lentivirus encoding miR-190, a microRNA targeting Neurod1, impairs adult neurogenesis and the abilities of mice to retain drug-associated contextual memory. Because adult neurogenesis is associated with contextual learning and memory, which is implicated in development of opioid tolerance, GIBH scientists hypothesized that NeuroD1 could be one of the many transcription factors involved in development of opioid tolerance.
During their studies, Prof Zheng and his group found that decrease in NeuroD1 activity impaired opioid antinociception immediately, whereas development of tolerance was decreased in a time-dependent manner. In addition, 190-vir and nd-vir driven by Nestin promoter affected development of tolerance but not antinociception. They concluded that decrease in NeuroD1 activity not only increases antinociceptive ED50 values immediately by affecting mature neurons but also impairs antinociceptive tolerance development in a time-dependent manner by influencing hippocampal contextual memory. These results are consistent with the critical contributions of NeuroD1 to both the morphologic and the functional maintenance of existing mature neurons and the generation of new neurons in the hippocampus.
In conclusion, Prof Zheng’s study established two distinct mechanisms used by Neurod1 to regulate opioid tolerance: increase in ED50 values and alteration of associative tolerance secondary to changes in contextual memory. Their studies also provided a new hypothesis to explain the prevailing observations that morphine has a higher ability to induce tolerance than fentanyl: their differential regulation of miR-190 level leads to differences in alterations in ED50 values.
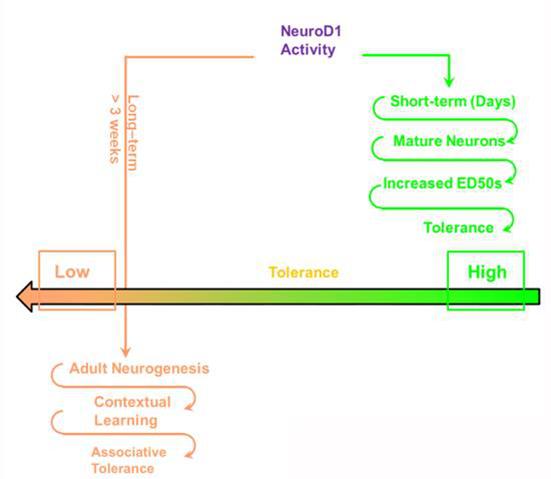
Neurod1 can modulate opioid antinociceptive tolerance via two distinct mechanisms.