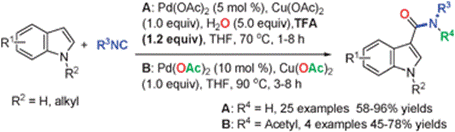Direct Carboxamidation of Indoles by Palladium-catalyzed C–H Activation and Isocyanide Insertion
Indole and its derivatives are a very important class of molecules existing widely in natural products and synthetic pharmaceutical agents with a broad range of bioactivities. Therefore, continuous efforts have been paid to their synthesis since more than a century ago. Recently, transition-metal-catalyzed C–H activation has received considerable interest of organic chemists and provides complementary and advantageous approaches to functionalized indoles.
Recently, a team led by Prof. Qiang Zhu, developed a first example of palladium-catalyzed carboxamidation of indoles including free (N–H) ones via C–H activation followed by isocyanide insertion, providing a general and efficient method for the synthesis of C3 carboxamidated indoles under mild conditions (Chem. Commun. 2012, 48, 3772–3774.). The reaction proceeds through a Pd-catalyzed sequential C–H activation–isocyanide insertion rather than Lewis acid promoted Friedel–Crafts reaction followed by oxidation of the imine intermediate. They found the presence of acid as additive is crucial for the product formation. Free (N–H)-indoles with diverse functional groups are well tolerable under the reaction conditions.
This work is generously supported by the National Science Foundation of China (21072190) .
.