GIBH scientists successfully carry out synthesis of imidazoles from ketones and aldehydes
In recent years, transition-metal-catalyzed direct C-H bond amination/amidation has been intensively studied due to the great importance of nitrogen-containing compounds in almost all areas of chemical research. The efficient construction of 2,4,5-trisubstituted imidazoles, through a copper-mediated three-component reaction involving ketones, aldehydes, and Me3SiN3, has been developed by Prof Zhu Qiang and his group.This work has been published by Chemical Communications (Chem.Commun.,2016,52,6467).
The scaffold of imidazole exists in many natural products and pharmaceutical agents with broad biological activities, such as antimicrobial, anti-neoplastic, anti-anxiety, and anti-inflammatory and consequently, much attention has been paid to their synthesis. The synthesis of densely substituted imidazoles is mainly focused on the three-component condensation of 1,2-diketones, aldehydes, and ammonia or modification of the existing imidazole core by transition-metal-catalyzed cross-coupling reactions. However, the development of a general procedure for imidazole synthesis starting from the readily available substance is still desirable.
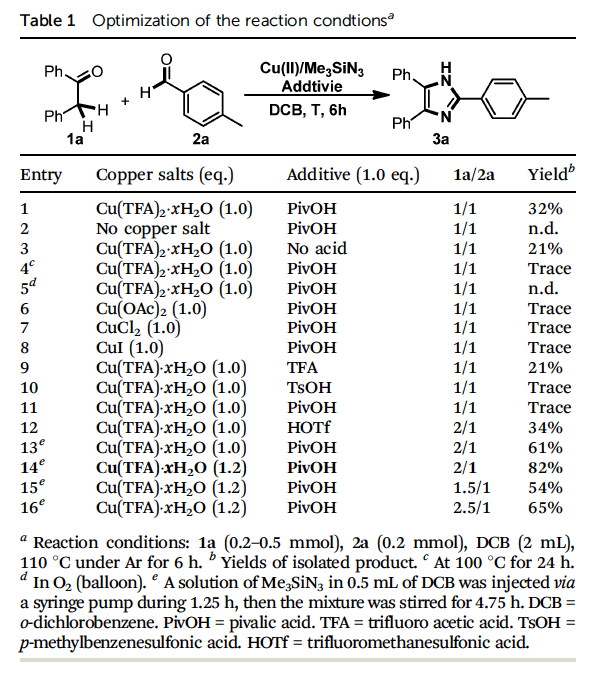
Prof Zhu and his group initiated the study by reacting 1,2-diphenylethanone 1a with p-methylbenzaldehyde 2a in the presence of Me3SiN3 under the reaction conditions they reported earlier. To their surprise, an imidazole derivative 3a, rather than the expected oxazole product, was isolated in 32% yield (entry 1, Table 1). Control experiments indicated that the copper salt was essential and PivOH was important for this transformation (entries 2 and 3). Screening of the copper species and acids revealed that the initial use of Cu(TFA)2?χH2O and PivOH was the best combination (entries 6-11). It was notable that when the reaction was performed in air or O2, the formation of 3a was completely inhibited, and 1,2-diketone, the oxidation product of 1a, was isolated as the major byproduct. Considering this side reaction, the amount of 1a was increased, and as a result, the isolated yield of 3a was slightly improved (entry 12). When a solution of Me3SiN3 in DCB was introduced into the reaction mixture via a syringe pump during 1.25h, the yield of 3a was improved significantly to 61% (entry 13). Finally, the novel imidazole synthesis reached a satisfactory level when 1.2 equiv. of Cu(TFA)2?χH2O was used (82%, entry 14). It was noteworthy that no by-product, derived from either Schmidt reaction or Jiao’s reaction through C-C bond cleavage, was detected.
In conclusion, Prof Zhu and his group developed an efficient method for the synthesis of tri-substituted imidazole staring from simple acetophenone derivatives and aldehydes, which are readily available. In this process, two nigroten atoms derived from Me3SiN3 were formally inserted into the target molecule by the formation of 4 C-N bonds. Azidation of the sp3 hybridized C-H bond is the key step for this multiple C-N bond-forming sequence, as suggested by experimental results and DFT calculations.