A transition-metal-free conversion of 2-arylindoles to 2-aminoarylphenones has been developed by scientists from GIBH
Nowadays much attention has been paid to chemoselective C-C bond cleavage followed by new C-C and/or C-heteroatom bond formation; since it enables a great number of readily available substances in chemical transformations. Recently, a transition-metal-free conversion of 2-arylindoles to 2-aminoarylphenones, using environmentally benign O2 as the sole oxidant, has been developed by Prof Zhu Qiang and his group. This novel oxidative dearomatization process involves cleavage of both C-C and C-N bonds followed by new C-C and C-O bond formation. The C2 carbon of an indole scaffold was released in the form of CO2. This work has been published by (Org.Chem.Front.,2016,3,364) Organic Chemistry Frontiers.
Prof Zhu and his group discovered an uncommon hypervalent iodine(m)-promoted tandem demethylenation/C-H cycloamination process of N-benzyl-2-aminopyridines via C-C and C-N bond cleavage. They presented a transformation of 2-arylindoles to unexpected products, which were identified as 2-aminoarylphenones, through dearomative C-C and C-N bond cleavage using environmentally benign oxygen as the sole oxidant. In this process, three chemical bonds (2C-C and 1C-N) are cleaved and one new C-C bond and one C-O bond are generated in one step.
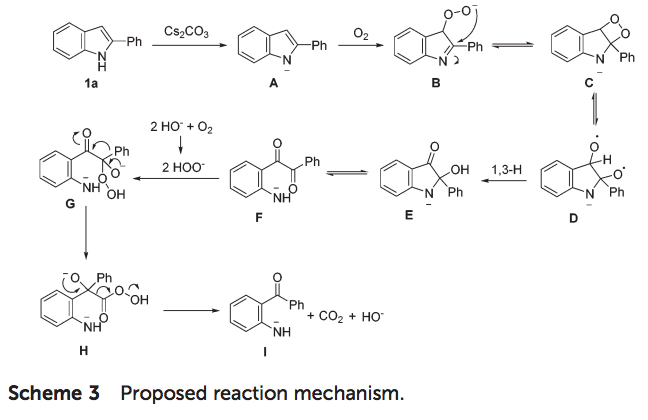
Based on the results they obtained, Prof Zhu and his group proposed a mechanistic pathway for this uncommon oxidative cleavage reaction (scheme 3). A peroxy anion B is formed by the deprotonated substrate in the presence of a base and oxygen. Then intramolecular nucleophilic attack on the C-N double bond results in a four-membered ring C. Subsequently, intermediate D is formed by a homolytic cleavage of the peroxy bond, followed by the 1,3-H shift to give intermediate E, which can be protonated to afford 3. Then, C-N bond cleavage in the aminoacetal moiety of E generates a diaryl-1,2-diketone F, which undergoes nucleophilic attack by the in situ-formed peroxy anion to afford intermediate G. Finally, aryl group migration and subsequent elimination of carbon dioxide give intermediate I, which is protonated to afford the final product.
In summary, Prof Zhu and his group have developed an unexpected, novel and environmentally benign method for the conversion of 2-arylindoles to 2-aminoarylphenones under transition-metal-free conditions using oxygen as the sole oxidant. The reaction proceeds through the cleavage of two C-C bonds and one C-N bond followed by new C-C and C-O bond formation. A reaction using 13C labeled 2-arylindole revealed that the C2 carbon of an indole scaffold was released in the form of CO2.