A Novel Extended PX Domain from Sorting Nexins Has Been Identified by GIBH Scientists
Endosome, a membrane-bound compartment inside eukaryotic cells, plays important roles in endocytic membrane transport pathway. Sorting nexins (SNXs), proteins containing a PX domain, are involved in several protein trafficking processes. As early as 2006, Dr Duanqing Pei’s group from GIBH found that over-expression of SNX10 induce endosome enlargement. In 2012, Dr Xiaodong Shu’s group found that SNX11, a close homologue of SNX10, can inhibit SNX10 induced endosome enlargement. SNX10 and SNX11 are believed to contain only PX domain, a membrane-binding domain. The mechanisms for their functions are still unclear.
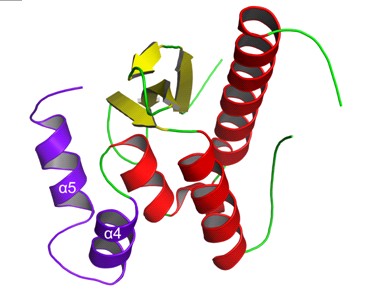
Recently, Dr Jinsong Liu’s group reported the crystal structure of SNX11, and revealed a novel PXe domain with two additional α-helices (α4 and α5) at the downstream of the conventional PX domain. Through collaborations with Drs. Shu and Pei, we found that SNX11-PXe domain also can inhibit SNX10 induced endosome enlargement like the full length SNX11. α4 and α5 are critical for the function of SNX11, but they are not required for the endosome targeting and protein stability, indicating that α4 and α5 may involve in partner interaction. Combined with the protein sequence analysis and previous results, we speculate that SNX10 also contain this novel PXe domain. This novel PXe domain may represent a structurally and functionally important subfamily of the PX domain.
This work had been published online by JBC (2013, doi: 10.1074/jbc.M112.449306). This work was supported by grants from the National Basic Research Program of China (973 Program) and the Natural Science Foundation of China.