Researchers Achieve the Asymmetric Synthesis of the Inherently Chiral Tribenzoannulene
Recently, the research group led by ZHU Qiang and LUO Shuang at the Guangzhou Institutes of Biomedicine and Health (GIBH), Chinese Academy of Sciences, achieved the enantioselective synthesis of various inherently chiral tribenzo[a,c,e][7]annulene derivatives by reacting palladium-catalyzed heptagonal oxime derivatives with bromo(chloro)benzyl. The relevant research findings were published under the title "Enantioselective synthesis of inherently chiral 9-benzylidene-9H-tribenzo[a,c,e][7]annulene and its application as a ligand platform"in Chem Catalysis.
Chirality is a fundamental geometric property of a significant proportion of molecules, and chiral molecules find extensive applications in pharmaceuticals, pesticides, and functional materials. Factors leading to the chirality of molecules typically include local structural features such as chiral centers, chiral axes, and chiral planes, as well as overall structural characteristics like helical structures. German chemist Böhmer, after an in-depth study of the chiral phenomena in calix[4]arene, introduced the concept of "inherent chirality", which is used to describe another type of overall structural feature in molecules beyond the commonly recognized chiral factors, specifically in molecules with certain rigid curved surfaces.
In the past few decades, extensive research has been conducted on central chirality, axial chirality, planar chirality, and helical chirality, resulting in many outstanding achievements. However, the study of the chiral characteristics of inherently chiral molecules, asymmetric synthesis, and their applications in areas such as biological activity and optical materials has lagged far behind. This is because the non-planar yet stable conformations of cyclic molecules in inherent chirality are often overlooked, being mistakenly considered as non-chiral flat molecules. Even if their non-planar conformations are acknowledged, these conformations are often thought to be dynamic and unstable.
After successfully achieving the asymmetric synthesis of two types of nitrogen-containing eight-membered ring inherently chiral compounds, in this study the researchers have once again accomplished the asymmetric synthesis of a more challenging all-carbon seven-membered ring, namely, inherently chiral tribenzoannulene derivatives.
The researchers reacted Pd-catalyzed heptagonal oxime derivatives with benzyl bromide to synthesize various inherently chiral tribenzoannulene derivatives with high yields and excellent enantioselectivity under mild conditions. In this process, the researchers revealed that the key steps determining the stereochemistry involve carbene migration insertion and β-hydrogen elimination to form the outer ring double bond by density functional theory calculations. Chirality is controlled through asymmetric β-hydrogen elimination, a rare example of generating chirality solely through β-hydrogen elimination.
Simultaneously, the researchers discovered that a chiral phosphine ligand 1 derived from one of the inherently chiral products exhibited excellent catalytic performance in asymmetric Tsuji-Trost reactions and 1,4-addition reactions, indicating that inherently chiral tribenzoannulene derivatives are outstanding scaffolds for designing new chiral ligands and catalysts. This once again demonstrates the great potential of long-overlooked inherent chirality in asymmetric synthesis and various other fields.
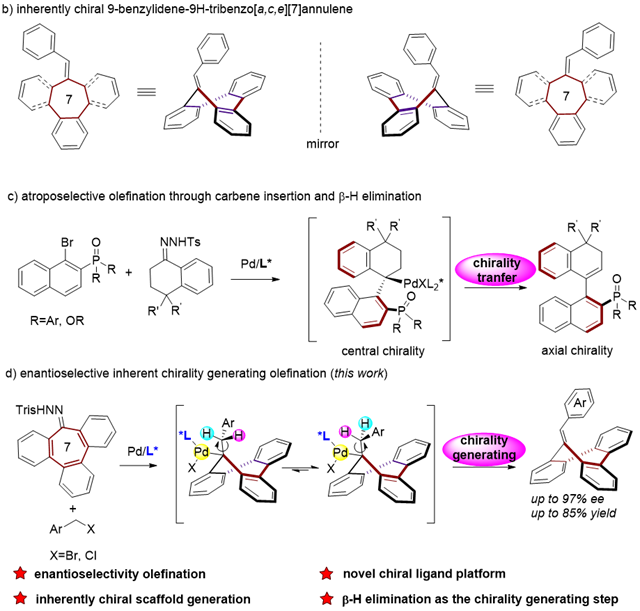
Palladium-Catalyzed Asymmetric Synthesis of Tribenzoannulene Derivatives.(Image by GIBH)
Contacts:
ZHU Qiang,Ph.D, Principal Investigator;
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou, China, 510530
Email:zhu_qiang@gibh.ac.cn
Attachment Download:
-
Contact
-
Reference
-
Related Article