GIBH Uncovers Novel Mechanism by Which BCL11B and NuRD Complex Cooperatively Maintain T-cell Fate
Adoptive cell therapy, particularly CAR-T and CAR-NK cell therapies, has emerged as a promising approach for cancer immunotherapy. These therapies have demonstrated remarkable success in treating hematologic malignancies. However, no CAR therapy has received FDA approval for the treatment of solid tumors. It prompts researchers to explore new strategies for targeting solid tumors effectively. Previous studies by Prof. LI Peng's team reported that inactivating BCL11B in mature human T cells induces their reprogramming into T-to-natural killer cells (ITNK cells). These ITNK cells exhibit both T cell and NK cell repertoires, which recognize and efficiently lyse both hematological and solid tumors in vitro and in vivo. Preliminary clinical trial results also suggest that patient-derived ITNK cells can effectively treat refractory and advanced solid tumors without causing any severe adverse effects. It demonstrates that ITNKs could serve as a new cell source for cancer immunotherapy. However, the potential antitumor efficacy of ITNK cells and the underlying mechanism are yet to be elucidated.
On September 22, a research, led by teams of Prof. LI Peng and Prof. LIU Xingguo from the Guangzhou Institutes of Biomedicine and Health(GIBH)of Chinese Academy of Sciences, has been published in the international journal EMBO Journal. Their study, titled“BCL11B and the NuRD complex cooperatively guard T-cell fate and inhibit OPA1-mediated mitochondrial fusion in T cells”, has uncovered that the NuRD complex and BCL11B cooperatively maintain T-cell fate through directly repressing NK-cell-associated transcription and indirectly through a metabolic-epigenetic axis, providing strategies to improve reprogramming efficiency and antitumor effects of ITNKs.
In this study, researchers showed that T cells upregulate the NK-cell associated receptors and transcription factors, lyse NK-cell targets, and are reprogrammed into NK-like cells (ITNKs) upon deletion of MTA2, MBD2, CHD4 or BCL11B. ITNKs increase OPA1 expression and exhibit characteristically elongated mitochondria with augmented oxidative phosphorylation (OXPHOS) activity. OPA1-mediated higher OXPHOS enhances cellular acetyl-CoA, thereby promoting the reprogramming efficiency and antitumor effects of ITNKs via regulating the acetylation of H3K27 modifications.
This research extends current knowledge on how mitochondrial dynamics regulate T cell fates. In addition, it reports alternative methods to derive ITNKs, potent tumor killer cells, from T cells and provides strategies to improve reprogramming efficiency and antitumor effects of BCL11B-deficient ITNKs.
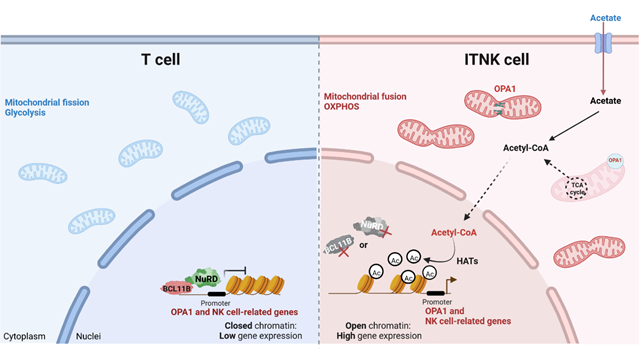
Fig. Schematic diagram of the mechanism by which BCL11B and the NuRD complex cooperatively maintain the T-cell fate (Image by GIBH)
Contacts:
Peng LI, Ph.D., Principal Investigator; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, China, 510530. Email: li_peng@gibh.ac.cn;
Xingguo LIU, Ph.D., Principal Investigator; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, China, 510530. Email: liu_xingguo@gibh.ac.cn.
Attachment Download:
-
Contact
-
Reference
-
Related Article