New Mechanism of HSC Homeostasis Regulation Revealed
Wang Jinyong's research team from the Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, and Cheng Hui/Cheng Tao's research team from the State Key Laboratory of Experimental Hematology, Institute of Hematology, Chinese Academy of Medical Sciences, collaborated and made new breakthroughs in the field of hematopoietic stem cell (HSCs) quiescence regulation. These new results were published online on December 10th in Haematologica, titled Loss of Nupr1 promotes engraftment by tuning the quiescence threshold of hematopoietic stem cell repository via regulating p53-checkpoint pathway. The study found that the transcription factor Nupr1 coordinates with p53 to form a signaling machinery regulating HSC quiescence and turnover rates. Deletion of Nupr1 activated the quiescence HSCs under homeostatic status, which conferred engraftment competitive advantage on HSCs. For the first time, the research team unveil the de novo role of Nupr1 in controlling HSC quiescence.
Hematopoietic stem cells (HSC) possess the capacity of self-renewal to maintain a stem cell pool, and the ability of differentiation potential to give rise of multi-lineage hematopoiesis. Under homeostasis, most HSCs are dominantly quiescent, and only a few cells proliferate to maintain hematopoietic homeostasis. In some stress states (such as excessive blood loss, radiation injury and chemotherapy), the HSCs can be rapidly activated for stress hematopoiesis on emergency conditions. When the body returns to homeostasis, the HSCs will re-enter into quiescence. This sophisticated homeostasis maintenance is regulated by a complex mechanism. To date, the underlying signaling regulatory network of HSC quiescence remains largely unknown.
In the beginning of the study, the research team found that Nupr1 was preferentially expressed in HSCs, when compared with the multipotent progenitors (MPPs) under homeostasis. In order to study the role of Nupr1 in the HSCs, the research team constructed a Nupr1 conditional knockout mouse model. Germline deletion of Nupr1 specifically activated dormant HSCs under homeostasis. Furthermore, loss of Nupr1 conferred repopulating advantages on HSCs, demonstrated by predominant Nupr1-/- HSCs and lineage cells under competitive setting with wild type HSCs in transplanted recipients. And in vitro expansion experiments showed that the Nupr1-/- HSCs proliferate more robustly than their wild type counterparts. Further transcriptome sequencing results revealed that the p53 signaling pathway of Nupr1-/- HSC was significantly down-regulated. The rescued expression of p53 offset the effects introduced by loss of Nupr1 in HSCs, indicating that Nupr1 regulates HSC homeostasis through p53 signaling pathway. This research unveil the de novo role of Nupr1 as an HSC quiescence-regulator, which provides insights into accelerating the engraftment efficacy of HSC transplantation by targeting the HSC quiescence-controlling network.
Wang Tongjie and Xia Chengxiang are the co-first authors of this paper, Wang Jinyong and Cheng Hui are the co-corresponding authors of this paper. This research was supported financially by the National Natural Science Foundation of China, Key Research & Development Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory, Strategic Priority Research Program of the Chinese Academy of Sciences, and CAS Key Research Program of Frontier Sciences.
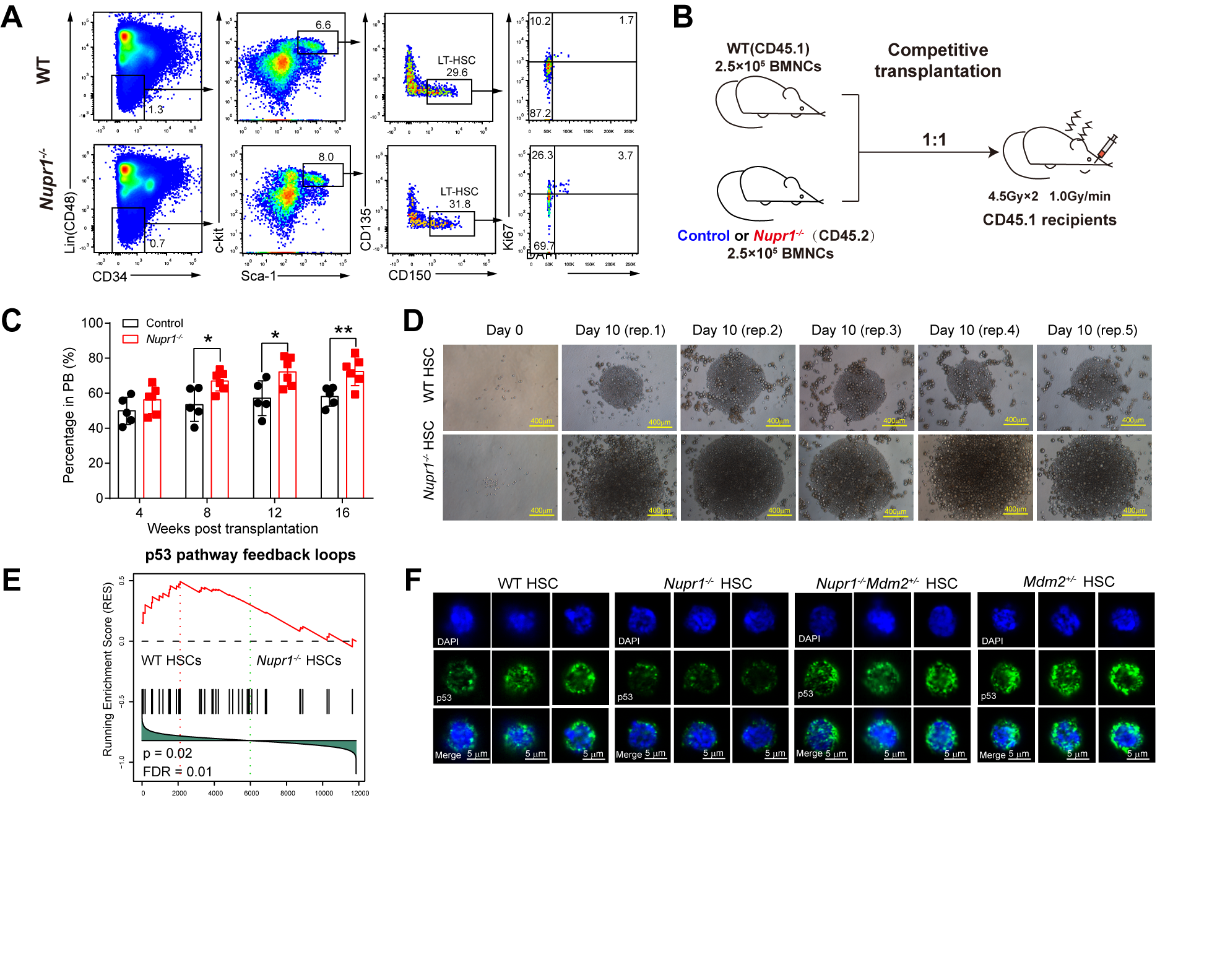
Loss of Nupr1 promotes engraftment by tuning the quiescence threshold of hematopoietic stem cell repository via regulating p53-checkpoint pathway
E-mai:wang_jinyong@gibh.ac.cn
Attachment Download:
-
Contact
-
Reference
-
Related Article