A new series of largazole analogues have been identified by GIBH scientists
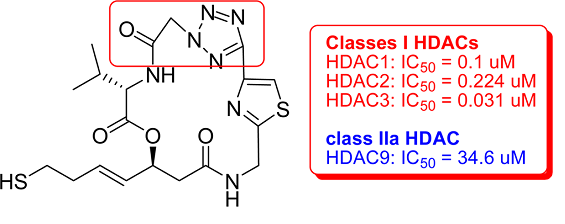
Recently, a research group led by Dr Sheng Jiang, Dr Zhengchao Tu and Dr Jinsong Liu has used computer-aided drug design principle and rational drug design method to successfully design and synthesis a new series of largazole analogues in which a 4-methylthiazoline moiety was replaced with a triazole and tetrazole ring, respectively. Compound 7 bearing a tetrazole ring was identified to show much better selectivity for HDAC1 over HDAC9 than largazole (10-fold). These results clearly indicate that the introduction of appropriate aromatic groups into the largazole skeleton is a useful optimizing tool for this unique class of anticancer agents. Various biological studies including inhibitory studies on different cancer cell lines, as well as inhibitory studies on metastatic tumors in animal models are currently in progress in this group. Their research has been published in ACS Medicinal Chemistry Letters in December 2012.



Attachment Download:
-
Contact
-
Reference
-
Related Article